| |
Prepared in collaboration with Jason White
Outline of Sections
 |
Summary of Basic Science and Clinical Information for Malaria (PDF)
(Chapter 9 from: Parasitic Diseases 5th Ed.) |
Section 1 |
|
Section 2 |
|
Section 3 |
|
3.1 |
|
3.2 |
|
Section 4 |
|
4.1 |
|
4.2 |
|
4.3 |
|
Section 5 |
|
5.1 |
|
5.2 |
|
5.3 |
|
5.4 |
|
5.5 |
|
5.6 |
|
5.7 |
|
5.7.1 |
|
Section 6 |
|
6.1 |
|
6.1.1 |
|
Section 7 |
|
7.1 |
|
Section 8 |
|
“Should there be rivers in the land, which drain off from the ground the stagnant water and the rain water, the people will be healthy and bright. But if there be no rivers, and the water that the people drink to be marshy, stagnant, and fenny, the physique of the people must show protruding bellies and enlarged spleens.”
Hippocrates
Airs, Waters, and Places
The above inscription is the first known record accurately describing aspects of the epidemiology of malaria, as well as presenting a few salient clinical features of the acute disease. Hippocrates observed and wrote about the three kinds of periodic fevers and chills associated with the synchronous forms of malaria. Today, physicians not only recite the Hippocratic Oath upon graduating from medical schools around the world, but they still refer to the fevers caused by malaria in a similar fashion. The word malaria is Latin, and means “bad air.” This lone infectious disease has been a perennial cause of human suffering and mortality, mainly throughout the tropical and sub-tropical regions of Africa, Asia, and South and Central America. According to the World Health Organization, more than 300 million diagnosed cases and 2 million deaths occur yearly. This represents a gross under-estimation of the problem, since millions more will most likely acquire the infection each year without ever realizing it.
There are over 400 species of the malarial parasite (Plasmodium spp.), many of which infect a wide variety of cold-and-warm-blooded animals, only four routinely infect humans. Each one of the latter is transmitted by the bite of an infected female Anopheles spp. mosquito. It follows then, that ecological alterations favoring the spread of these insects also facilitate the spread of the infection wherever malaria occurs. To get some idea of the complexity of the ecological differences among the numerous malaria endemic zones, one must consider at least four different, yet related, aspects: the host, the insect vectors, the parasites, and the physical conditions under which transmission occurs. Integration of these seemingly disparate subject areas into a unified view with respect to geographic locale is essential to begin identifying environmental factors that might be taken advantage of for the purpose of controlling the spread of the parasite.
As mentioned, the Greeks were the first to write about malaria. Even earlier, the Egyptians made numerous references to it in their hieroglyphs. However, it wasn’t until late in the 18th century that serious inroads into its etiology were made. Laveran, working on bird malaria in north Africa, is given credit for first observing the living parasite inside red blood cells, yet his most exciting discovery was in microscopically observing the development of male sex cells of the parasite, referred to as exflagellation, in a drop of infected bird blood. These important findings earned for him a Nobel Prize in medicine.
Yuri Romanovsky, working in Russia, independently developed the stains needed to clearly identify the parasites in blood smears, allowing anyone with the interest and a microscope to become involved in malaria research. In 1897-1898, Ronald Ross, a future Nobel laureate, discovered during his work in India that female culicine mosquitoes were the transmitters and definitive hosts for bird malaria (i.e., they harbored the sexual stages of the parasite). Ross demonstrated how transmission of the parasite occurred by successfully infecting twenty-one out of twenty-eight healthy sparrows and other passerine birds employing mosquitoes that had previously fed on infected birds. He found the parasites in the stomach of the infected mosquitoes, and shortly thereafter discovered the sporozites (the infective stage) in their salivary glands. Ross speculated, based upon his fundamental findings, that a single experiment using red cells taken from patients infected with Plasmodium falciparum and dapple-winged, anophelene mosquitoes would establish the existence of a similar cycle for human malaria.
Grassi, Bignami, and Bastianelli, working in Italy, used human volunteers and confirmed Ross’s suspicions, and published their findings in a series of seminal papers in 1898 and 1899.
Malaria is caused by a protozoan belonging to the genus Plasmodium. Although there are over 400 species, four routinely cause disease in humans: Plasmodium falciparum, P. vivax, P. ovale, and P. malariae. While they all belong to the same genus, each species behaves quite differently in most aspects of their biology within the human and mosquito host. They also vary with respect to geographic distribution. P. falciparum is found in most tropical regions throughout the world, and is the most dangerous of the four in terms of both its lethality and morbidity. Relapses cannot occur with this species. In contrast, relapses due to P. vivax can routinely occur due to a latency period, during which time the parasites in the infected hepatocytes remain dormant. The longevity of relapse is apparently dependent on the particular geographical region in which the organism is found. P. vivax is prevalent in many sub-tropical zones, as well as in the tropics. P. ovale is similar to P. vivax in its biology, but is found primarily in West Africa.
All species of plasmodia undergo two forms of replication, asexual and sexual. During the asexual stage, the organism enters the bloodstream of the host through the bite of an infected female anophelene mosquito. At this point, it is spindle shaped and motile, and termed the sporozite. After being introduced into the human intermediate host, it enters the bloodstream and is carried to the liver. There, it penetrates an hepatocyte and undergoes growth and multiplication. In the case of P. vivax and P. ovale, some sporozoites transform to the dormant hypnozoite instead, and remain so for varying lengths of time up to 5 years. This stage is responsible for relapses when it re-enters its developmental cycle. Multiple cycles of division result in the production of thousands of new parasites (merozoites). As the result, the host cell ruptures, releasing them into the bloodstream. They can no longer return to the liver and infect hepatocytes, and now must infect red blood cells in order to remain viable.
Merozoites enter the bloodstream, attach to and penetrate red cells. This begins the erythrocytic stage of the life cycle. Plasmodia ingest, and then digest hemoglobin, thereby acquiring amino acids needed for protein synthesis. They discard heme and a small peptide side chain, creating a compound known as hemazoin. Hemazoin molecules become stacked, one on top of the other, inside the food vacuole with the aid of a specialized enzyme, forming a crystalline deposit. The drug chloroquine interferes with that process by inhibiting the stacking enzyme. The parasites divide and lyse open the red blood cell, re-entering the bloodstream as merozoites. Hemazoin accumulates inside the host cell and is also released at the time of cell lysis. This material is known as “malarial pigment” and is visible under the light microscope.
Plasmodia also reproduce sexually within the mosquito, the definitive host. Undifferentiated merozoites in the bloodstream transform into pre-sex stages termed microgametocytes (male) and macrogametocytes (female), and are subsequently ingested. Within the lumen of the insect stomach, haploid male gametes, the stage first observed by Laveran, are produced, and fuse with female gametes to form a diploid zygote that eventually elongates and differentiates into an ookinete. The ookinete then penetrates the mosquito’s stomach wall and develops into an oocyst. The oocyst produces thousands of haploid sporozoites, and they penetrate out of the oocyst wall, entering the haemocoel of the insect. The circulation carries them to the salivary glands, where they penetrate into the lumen and tissue of the gland, awaiting the insect’s next blood meal.
3.2 |
Ecology of Transmission |
|
Unlike some diseases, which flourish under one particular set of environmental conditions, the environmental factors that contribute to the transmission of malaria vary greatly from one ecological zone to another. Of all of these, the two most important ones are temperature and humidity.
Organisms cease to develop in the mosquito when the temperature falls below 16°C. At 20°-30°C, the parasites develop optimally in the vector. High humidity prolongs the life of the vector and transmission is extended under these conditions. In the human intermediate host, the parasite must function at 37°C or higher, since the infection induces a significant rise in core temperature during the height of the infection.
4 |
Medical Ecology of Malaria, Part I: Biology of Vector Species |
All mosquitoes require water to complete their life cycle. Development is of the complete type, and consists of four stages: egg, larva, pupa, and the adult. While between 200-1,000 eggs can be laid, the quantity is influenced by the amount of blood taken in. Once egg laying is complete, the female retires to an area where it may once again take another blood meal.
Both male and female mosquitoes feed exclusively on nectar and fruit juices. The female takes in blood as a rich source of protein in order to produce her clutch of eggs. The female often takes her first blood meal the night after she emerges from the pupal stage. Blood feeding usually occurs between dusk and dawn, although some species feed during daylight hours in densely shaded woodland areas. Anopheles spp. frequently rest inside houses and other domestic dwellings. Females commonly enter a house after dark, take a blood meal, and then find a place to rest during daylight hours.
Before the mosquito ingests blood, it must first find a host. Mosquitoes possess a highly developed set of host-detection organs that they use to sense color, light, touch, and temperature, as well as the presence of CO2. They use all of these sensory inputs to locate a host. When a mosquito has found an appropriate host on which to feed, it uses its two pairs of mandibles and maxillae to penetrate the skin of the animal and pierce a capillary vessel, allowing easy access to blood. It injects numerous biologically active compounds that cause the vessels to dilate, blood to flow without coagulation, and to prevent the host from detecting her presence.
Mosquitoes ingest blood through the hypophaynx by the action of a pharyngeal pump, and digestion of the meal takes place in the midgut and stomach. Depending on the size of the female, between 1.3 and 3.0 µl of blood is ingested. The digestive system is also the same part of the mosquito’s body where important differentiation events and growth of the malaria parasite occur.
4.2 |
Ecology of Transmission |
|
Each environmental change in the mosquito habitat, whether occurring as the result of a natural process or through human intervention, rearranges the ecological landscape in which these vectors breed. Every Anopheles spp. occupies a particular ecological niche that is genetically determined. Changes in temperature, humidity, altitude, population density of humans, and deforestation are just a few ecological factors that each play essential parts in the transmission of malaria.
Temperature and humidity have a direct effect on the longevity of the mosquito. Each species can thrive at an optimal level as a result of ecological adaptation. The spread of malaria requires that conditions are favorable for the survival of both the mosquito and the parasite. Temperatures from approximately 21°-32°C and a relative humidity of at least 60% are most conducive for maintenance of transmission.
Mosquito density is conveniently measured in terms of the number of female mosquitoes per human inhabitant of the area. Thus, malaria transmission is proportional to mosquito density. Mosquito longevity affects malaria transmission, because it takes time (approximately 1 week) for the parasite to develop. Typically, female mosquitoes live 2.5-3 weeks. The minimum length of development is temperature dependent in all mosquito habitats, even the tropics.
Altitude is significant in determining the distribution of malaria and its seasonal impact on many regions of the world. In Africa, for example, altitudes above 1,000-1,500 m are considered safe from malaria. However, it must be cautioned that with continuing global climate change, these figures may change, extending the range of mosquitoes well above those altitudes as ambient temperatures rise.
One of the most disruptive changes affecting mosquito populations is deforestation. When forest is cleared, erosion of the soil occurs, stripping away nutrients. It may take up to 50 years or more before a deforested area in the tropics returns to normal. During this time, cleared tropical forest is typically converted into grazing pastures, agricultural plots, and human settlements. These ecological disturbances allow for the proliferation of mosquitoes that prefer human habitation to natural settings.
In Trinidad, following deforestation during the 1940’s, Erythrina micropteryx (Immortelle) trees were imported from Peru to shade the cocoa trees. Bromeliads (epiphytes) began to grow on them. This in turn, provided a breeding habitat for An. bellator and An. homunculus, while also increasing the opportunity for effective transmission of P. malaria in rural areas. As a result, a large malaria epidemic occurred, because the water-collecting bromeliads are the preferred breeding site for An. bellator. When the bromeliads were eventually removed by spraying dilute solutions of copper sulfate into them, the prevalence of malaria was greatly reduced. This illustration shows how a simple change in the ecological niche, such as the importation of immortelle trees, created a favorable transmission environment where none existed before. Fortunately, in this case, a solution was forthcoming.
Construction of water control projects can also lead to shifts in vector mosquito populations. Reservoirs, irrigation canals, and dams are closely associated with the increase of a variety of parasitic diseases that are water dependent. Throughout the world, especially in developing countries, dams and other related water projects continue to be planned, constructed, and operated to meet human needs such as drinking water, energy generation, and agricultural production.
For most countries, dams are a crucial part of economic and social development, and represent a double-edged sword. The potential for dams to alleviate poverty significantly contributes to the enhancement of human health, and simultaneously increases the likelihood of human infection due to schistosomiasis, malaria, dysentery, and river blindness. During the construction of dams and canals, excavation pits provide temporary breeding sites for mosquitos. Such negative consequences are not only the direct result of encroachment, but are also due to the establishment of new strains of malaria brought in by migrant labors working on these dam sites. In these situations, disease transmission occurs at the ecotone.
4.3 |
Ecology of Mosquito Breeding Sites |
|
The breeding sites of infected mosquitoes vary greatly with regards to species. Some prefer clear water, inhabiting the edges of streams, while others thrive in irrigation ditches and reservoirs. Some species require extensive vegetative cover, preferring swamps and other permanent bodies of water laden with dissolved organic matter. Mosquito breeding sites are found anywhere fresh water collects. In fact, there is a direct correlation between the availability of water and the frequency in which mosquitoes feed on humans. Permanent natural bodies of water, such as swamps, serve as unique breeding grounds. In the tropics, as mentioned, mosquito breeding sites have emerged due to construction of dams and canals. Many of these sites develop into zones of transmission due to the concomitant increase of human populations moving to these areas. The number of possible breeding sites is extensive, and describing a few more of them will help to illustrate the difficulty in finding a common solution to control of malaria transmission by limiting mosquito populations.
Ecological disturbance as a direct result of human activity may also increase the number of breeding sites. Road building and maintenance projects often impede drainage of runoff from rainfall. Clogged drainage ditches along roads left by logging and construction activities are ideal places for floodwater mosquitoes. Around the house, objects such as empty cans, discarded tires, potted plants, and similar objects collect rainwater and allow mosquitoes to breed within the limits of human habitation. Many of these ecological settings can be identified by remote sensing from outer space.
Satellites acquire information through a variety of modes, helping to identify potential breeding sites. Vegetation with unique reflection spectra, and human settlements that emit weak electromagnetic radiation signals are key to making the correlation between potential breeding sites adjacent to human habitation, and the potential spread of malaria.
Remote sensing technologies have allowed medical ecologists to view vast areas of malaria transmission, and has provided a new and important tool for mapping the breeding habitats of infected mosquitoes, predicting densities of vector species, and even developing risk maps for malaria transmission.
5 |
Specific Vector Biology |
|
The biting behavior of the vector is dependent on several factors. To be an effective transmitter of malaria, the mosquito must fulfill certain requirements. These include, but are not limited to, their frequency of taking a blood meal, the mean longevity of the breeding season, and the density of vectors in relation to human population density.
Most Anopheles spp. are nocturnal in their blood meal feeding habits. Some species, such as An. albimanus found in Central and South America, bite mainly indoors from sunset to 21.00 hours. In contrast, in Africa, the An. gambiae species, also an indoors feeder, bite mainly after 23.00 hours. Feeding preferences vary considerably with respect to time, place, and host species availability. Anopheles spp. feed on a wide variety of hosts, including birds, cows, pigs, and all primate species. Most of these mosquitoes are not exclusive in regards to their preferred host species. For example, An. culicifacies, an important malaria vector throughout most of tropical India, commonly feeds on cattle as well as humans. In Africa, however, An. gambiae feeds rarely on cattle, and more frequently on humans. This is one of the reasons why An. gambiae is a more efficient malaria vector than An. culicifacies when they are found together in the same ecological setting with human populations.
5.1 |
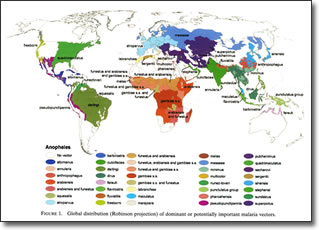
Geographic Distribution of Mosquito Vectors of Human Malaria |
|
North America (and Mexico)
- An. freeborni
- An. quadrimaculatus
- An. pseudopunctipennis
|
Central America
- An. albimanus
- An. aquasalis
- An. darlingi
|
South America
- An. nigrrimus
- An. albimanus
- An. aquasalis
- An. darlingi
- An. nuneztovari
- An. anthrophagus
- An. pseudopunctipennis
|
North Eurasia
- An. stevensi
- An. atroparvus
- An. fluviatillis
|
Mediterranean
- An. atroparvus
- An. labranchiae
- An. superpictus
- An. maculapennis
|
Afro-Arabian
- An. phaeroencis
- An. sergentii
|
Afrotropical
- An. arabiensis
- An. funestus
- An. gambiae
|
Indo-Iranian
- An. culcifacies
- An. fluviatilis
- An. stevensi
|
Indo-Chinese Hills
|
Malaysia
- An. aconitus
- An. balabacensis
- An. dirus
- An. donaldi
- An. flavirostris
- An. letifer
- An. maculates
- An. minimus
- An. sundaicus
|
Chinese
|
Australasian
- An. punctulatus
- An. faraudi
|
Originating in Africa, the Anopheles gambiae complex of mosquitoes include the most efficient malaria vectors on Earth. This is because they have adapted well to human habitation, and feed almost exclusively on human blood for egg production. It has a wide distribution, and usually occurs in large numbers wherever found. It is also highly susceptible to the parasite. The female bites mainly at night, but in several studies, 12% of bites occurred after sunrise. An. gambiae lay eggs in a wide variety of aquatic environments. Some breed in small pools that are partially or completely exposed to the sun, while others prefer to breed in shaded stagnant pools, or even in slow moving water. They have been captured from water-filled holes of rocks, coral outcrops, trees, and stumps of felled banana trees. Wherever agriculture or gardening activities result in the collection of significant amounts of stagnant water, An. gambiae is there to take advantage. Because of its high degree of ecological adaptability, this vector species has become the dominant one throughout Africa. An investigation by the World Health Organization showed the following regarding An. gambiae breeding sites.
Under laboratory conditions, this species carries out normal development when the pH varies as much as from 4.0 to 7.8, as long as there is sufficient phytoplankton and zooplankton for it to consume. The maximum temperature at which An. gambiae larvae can survive is 41°C. Rarely does this temperature occur in nature, even in the intense heat of equatorial Africa.
5.3 |
Anopheles quadrimaculatus |
|
An. quadrimaculatus is the dominant vector in the southeastern half of the United States. Malaria transmission is rare, however, due to lack of the presence of the parasite. Introduced infections are common during the height of the tourist season, when many travelers return from endemic tropical regions with sub-clinical cases of malaria, and serve as foci of domestic infections. During the early history of the United States, malaria was common, and did not completely disappear until the early 1950s.
Anopheles quadrimaculatus is essentially a pond breeder, wherever sunshine and emerging vegetation are available. Larvae are found along the littoral zone. It also breeds in rivers or swamps where conditions are similar to those found in ponds. Unlike An. gambiae, An. quadrimaculatus prefers slightly alkaline water. An. quadrimaculatus enters occupied houses freely and rests within the darker corners in the daytime. They are even found in large numbers in stables and other animal shelters. Moreover, they are sometimes exceedingly abundant in tree holes, under bridges, and in caves.
The ecology of An. quadrimaculatus is very similar to that of other Anopheles spp. One unique feature of its ecological preferences is that it will not lay eggs at temperatures below 12°C.
An. freeborni is an important malaria vector in the western United States. Specifically, it is found west of the Continental Divide, in southern British Columbia, throughout California, Arizona, and east of the divide in southern Colorado and New Mexico. This species enters homes and animal shelters by preference, biting throughout dusk and dawn. From night to night, it migrates from one shelter to another, doing most of its traveling during the evening or morning hours.
The mosquito prefers semi-arid regions of seasonal rainfalls. In the fall, at the end of the dry season, females migrate for long distances (usually ten to twelve miles away from their breeding sites) to reside in homes, cellars, and similar locations. An. freeborni prefers permanent or semi-permanent water surfaces exposed to sunlight, with some shade provided by emerging vegetation or algae. However, the winter is also conducive to malaria transmission in heated homes. When ideal temperature and humidity conditions are maintained (26°C and 80% humidity), continuous breeding is possible. An. freeborni feeds on the lower extremeties. During February, they emerge from their winter shelters on warm sunny days and bite in full daylight. Moreover, the density of adult An. freeborni builds up to a high peak in late May and early June in the hot interior valleys. As temperatures increase with corresponding decreases in humidity, the lifespan of the adult also decreases.
In South Asia, there is little transmission of malaria in built-up areas of this region because most of the vector species have not adapted to urban conditions. On the other hand, An. stevensi, which feeds mainly on humans, is an exception that is responsible for malaria transmission in some of the largest cities in India (Bombay, Bangalore, and Lucknow). This mosquito is also an important vector in rural regions of western and northwestern India.
In moderately populated areas, A. stevensi larvae have been found in wells, cisterns, fountains, garden tanks, and tubs. They can also be found in various household receptacles. In rural areas, they breed in pools, streambeds, irrigation channels, and springs. Adults are strong flyers, but the effective range does not exceed 0.5 miles in most urban settings.
5.6 |
Anopheles punctulatus |
|
A dominant Anopheles spp. has been emerging throughout Asia, the Anopheles punctulatus complex, and consists of Anopheles punctulatus, An. koliensis, and at least three species of An. farauti. Members of this complex are important vectors of human malaria and periodic Bancroftian filariasis throughout Asia. An. punctulatus biting activity occurs nocturnally, usually peaking at approximately 10:00 p.m. These related species of mosquitoes are typically larger and more robust than those found at lower elevations. This makes them better vectors because of the relatively large blood meals that they take.
An. Punctulatus is the dominant vector of Papua New Guinea. This species is associated with varying rainfall patterns and can survive in causal surface waters. In Papua New Guinea, the clearing of native vegetation and poorly maintained irrigation ditches around houses in that tropical region creates and maintains many breeding conditions for these vectors.
5.7 |
Case Study: The Emergence of Anopheles marajoara as a New Dominant Malaria Vector |
|
In Amapá state in the northeastern region of Amazonia, a new neotropical species of anopheles has been identified as an emerging dominant malaria vector. In five collections taken from three replicated sites near the city of Macapá, Amapá state in 1996-97, An. marajoara was found in greater abundance during the peak transmission months of June-August and November-December than the previously dominant vector in the area, An. darlingi. In addition, P. falciparum and P. vivax were also found with significantly higher frequency in the An. marajoara population than An. darlingi. Data from the Fundacao Nacional de Saude also indicated the occurrence of new malaria cases during months when no An. darlingi were collected. Instead, in Amapá state, a high prevalence of An. marajoara was demonstrated. There are an estimated 500,000 yearly malaria cases in Brazil, for which, in the past, the primary vector has been An. darlingi. Over the last few years, ELISA, in conjunction with microscopic examination of mosquito stomachs, researchers have identified new set of neo-tropical species as potentially important malaria vectors. The species complex An. albitarsis s.l. consists of 4 species: An. albitarsis s.s., An. deaneorum, An. marajoara, and An. albitarsis sp. B.
5.7.1 |
Ecological and Environmental Factors Leading to Emergence of a New Vector |
|
Three ecological factors led to the emergence of An. marajoara as the new dominant vector in northeastern Amazonia: recent alterations in land use in Amapá state; human migration, population influx and invasion of primary mosquito breeding sites; and An. marajoara’s more anthropophilic tendencies than An. darlingi. At the time the tests were conducted (1990-1998), the capital city of Amapá, Macapá, underwent significant industrial developments, including the construction of roads through several forests, and was established as a duty free zone. Both changes generated a significantly larger increase in human traffic through the area than before, and also attracted an increased influx of immigrants. Many immigrant populations of adjacent states were already infected with malaria, and consequently the percentage of the population infected in Amapá state rose from 4.3% to 15% between 1990 and 1998.
Furthermore, the advent of roads through sections of virgin forest, and urban development, has resulted in encroachment into primary mosquito breeding sites. Large, undisturbed areas of unshaded, unpolluted and stagnant water sources were disrupted, and many forested areas were cleared. These changes have selected against An. darlingi, and inadvertently created new sites for other species, such as An. marajoara, to emerge.
It is likely that these environmental changes led directly to the population increase of An. marajoara and its new position as the primary malaria vector in that region. It was previously believed that malaria could be limited by controlling An. darlingi. Unfortunately this is no longer the case, as there are now more factors to consider.
6 |
Medical Ecology of Malaria, Part II: Human Ecology and Its Influence on Malaria Transmission |
|
As alluded to, human populations within a given region can have significant effects on the transmission of a disease. As people move and change environments, they introduce new technologies to the area and increase their own likelihood of acquiring or spreading an infectious disease. The most important aspect of malaria as it affects the human community is its endemicity, or degree of prevalence. This covers a wide range of possibilities from sporadic cases to hyperendemic situations in which few people escape infection.
In some regions, malaria occurs in massive epidemics, affecting people of all ages and causing temporary social disruption. This pattern, alternating between large epidemics and “scare” malaria, is referred to as unstable malaria. At the other extreme is stable malaria, where transmission is continuous or seasonal, and mosquitoes constantly infect and re-infect the human population.
Malaria transmission can be divided into three environmental regions: seasonal (stable), continuous (stable), and intermittent (unstable). Unstable and stable malaria not only differ in their mortality rates and public-health effects, they also vary in their responses to control strategies. The same control scheme may have very different outcomes, depending upon the area in question. These differences are biologically determined, and must be understood if stopping the spread of the disease is the desired outcome.
6.1 |
Continuous, Seasonal, and Intermittent Malaria |
|
The seasonal incidence of malaria is ordinarily represented by a wave of infection in the spring and by an autumnal wave of greater amplitude. The amplitude of seasonal malaria is not constant in all parts of the world. In fact, seasonal malaria even in the tropics has seasonal oscillations. However, seasonal malaria in the tropics occurs with remarkable regularity at the same time each year. In the subtropical and temperate zones, the situation is different, though nevertheless as complex. In these zones, the development of malaria varies from year to year.
In most parts of the world, malaria presents a definable seasonal periodicity due to a seasonal increase in the number of relapses in the community, together with an increase in the frequency of transmission. For example, Malaysia has two seasonal waves of malaria. In the spring the wave reaches its maximum in May, while an autumnal wave reaches its maximum in October or November.
Malaria is seasonal in places where climatic conditions allow for the periodic or occasional development of parasites and vectors. Indigenous populations do not have enough time between peak times and transmission to develop a proper immunity. Specific groups of people at risk for contracting the disease are harder to define and the mortality rate during epidemics can be very high. Usually, epidemics are linked to the absence, decrease or loss of collective immunity.
6.1.1 |
Intermittent Malaria Conditions |
|
In some transmission zones, favorable climatic conditions exist all year for both vector and parasite. There are no significant inter-annual fluctuations, although some seasonal fluctuations may exist. The infection rate is high and can be maintained by small populations of mosquitoes, making malaria virtually impossible to eradicate in some ecological settings. Indigenous populations build up low levels of immunity that protect them from high mortality rates. As a result, most deaths from malaria occur in infants and children under five.
7 |
Medical Ecology of Malaria, Part III: Control Strategies |
|
Control of malaria may eventually prove tougher to implement than either HIV or tuberculosis because of its distinct life-cycle stages and genetic complexity, the latter of which allows plasmodium to rapidly develop drug resistance. A single infected individual can serve as a focal point of infection for hundreds of other individuals within months.
Various insecticides have been developed employing a bacterial-derived toxin that is effective against the larval stage of the mosquito. Known as BTi (Bacillus thuringiensis israeliensis) With bacterial insecticides, large bodies of water can be treated with bacteria containing BTi. The advantage of this method is that it is harmless to humans, other animals, fish, and many, but not all beneficial insects. One disadvantage is the bacterial toxin’s limited span of effectiveness. In addition, BTi is only effective in killing larvae, and not eggs or pupae.
Chemical-based insecticides are another strategy for controlling mosquitoes. Petroleum oils, chlorinated hydrocarbons and carbonates are a few examples, and are still widely used to interfere with the life cycle of the mosquito. These insecticides are either applied to mosquitoes directly, or are released into the surrounding air space. Dichlorodiphenyltrichloroethane (DDT) and hexachlorocychlohexane (HCH) have been effective malaria controlling agents. Their efficacy has served as a stepping-stone in the fight against the disease.
DDT and HCH are low in toxicity for humans. There is no evidence to suggest that people whose homes are sprayed with these chemicals are at any risk of being harmed. On the other hand, while DDT is not harmful to humans, it is toxic to animals such as cats, chickens, predatory birds and fish. This is not the only disadvantage to chemical insecticides. DDT also has a short span of activity when used as a larvicide. Heavy rain and mud can dilute the effects of the chemical thus making it inactive.
7.1 |
Development of Resistance to Biological and Chemical Insecticides |
|
Over the years mosquitoes and other noxious insects have been selected for resistance to DDT and other insecticides. As a result, during the 1970’s, malaria control through insecticide use declined, while the number of reported malaria cases rose by 30%. Multiple factors influence resistance to insecticides, including method of application (the particular type of insecticide and its method of application), the size of the insect population, and especially genetics (the presence of specific resistant genes and their frequency). Heterozygous insects with genes for resistance often mate with other resistant heterozygous mosquitoes to create progeny with an even higher degree of resistance.
Resistance to antimalarial drugs in the parasites is now common throughout most parts of the tropics. Antimalarial drugs prevent the development of the parasite in the liver or attack the merozoite in the red blood cell. Common drugs, including quinine, chloroquine, and tetracyclines, are regularly prescribed to the millions of people who live and travel in regions where malaria is endemic.
Thailand experienced a 20% reduction in the incidence of malaria, from 198,000 in 1991 to 152,000 in 1992. Despite this positive trend, however, by 1998, 349,000 cases were recorded. In high transmission areas such as in the provinces of Trat (bordering Cambodia) and Tak (bordering Myanmar), multiple drug resistant P. falciparum strains have clearly spread and are now resistant to chloroquine and sulfadoxine-pyrimethamine. In the last two years, P. falciparum has become resistant to mefloquine, as well. The cure rate with this drug has now fallen to below 50% in these border areas.
By the end of 1983, chloroquine resistant strains were reported from the New Hebrides in the southwest Pacific, through Papua New Guinea, the Philippines, Indonesia, and into the Indo-Chinese peninsula, and nearly halfway across the Indian subcontinent. In parts of Southeast Asia, P. falciparum resistant to almost all antimalarial drugs now dominate the clinical cases, and chloroquine-resistant P. vivax strains have also emerged. In Africa, chloroquine resistance is widespread and resistance to sulfadoxine is frequently detected.
Scientists around the world are hard at work to develop an effective vaccine designed to prevent mortality due to severe infection. Due to the extreme complexity of the parasite, to date, prospects for a practical malaria vaccine seem remote. Therefore, the only low tech option available to those living in endemic areas seems to be the avoidance of mosquitoes using repellants and bed netting.
Bangs, M.J. 1996. “Malaria transmission by Anopheles punctulatus in the highlands of Irian Jaya, Indonesia”. Annals of Tropical Medicine and Parasitology. Vol. 90, No. 1.
Bould, M.F. Malariology: A Comprehensive Survey of all Aspects of this Group of Disease from a Global Environment. W.B. Saunders Company: Philadelphia, 1949.
Bradley, D.J. 1992. “Malaria: Old Infections, Changing Epidemiology”. Health Transition Review. Vol. 02, Supplementary Issue.
Burkot, Thomas. 1988. “Mixed Blood Feeding by the Malaria Vectors in the Anopheles punctulatus Complex”. J Med. Entomology. Jul: 25(4). pp. 205-213.
Chadee, D.D. 1999. “Spatial and Temporal patterns of Imported malaria Cases and Local Transmission in Trinidad.” American Journal of Tropical Medicine and Hygiene. Vol. 61, No. 4. pp. 513-517.
Connor S.J., Thomson M.C., 2001. “The development of malaria early warning systems for Africa”. Trends in Parasitology. pp. 438-444.
Despommier, D., Gwadz R.W., Hotez P.J., Knirsch C.A. Parasitic Diseases (4th edition). Apple Trees Publications LLC: New York, 2000.
Geller N., Graczyk T.K., Patz J.A., Vittor A.Y. 2000. “Effects of environmental change on emerging parasitic diseases”. International Jour. for Parasitology. pp. 1395-1405.
Greenwood, B., Mutabingwa, T. 2002. “Malaria in 2002”. Nature. Vol. 415. pp. 670-672.
Indian Council of Medical Research. 2000. “Remotes Sensing: A Visionary Tool in Malaria Epidemiology”. Vol. 30, No. 11.
Institute of Health. Malaria: Obstacles and Opportunities. National Academy Press: Washington D.C., 1991.
Jobin, W. Dams and Disease: Ecological Design and Health Impacts of Large Dams, Canals, and Irrigation Systems. E & FN SPON: London & New York, 1999.
Komp, William. 1992. “Notes on mosquitoes from an area of endemic yellow fever in Columbia”. Entomological Society of Washington. Vol. 58, Feb.
Service, M.W. Medical Entomology. Chapman & Hall, 1997.
Shell, E.R. 1997. “The Resurgence of a Deadly Disease”. The Atlantic Online.
[Back To Top] |
|