| |
Prepared in collaboration with Ethan Bodle
Outline of Sections
 |
To learn more about the original epidemic in the United States, click here. |
Section 1 |
|
Section 2 |
|
Section 3 |
|
Section 4 |
|
Section 5 |
|
Section 6 |
|
Section 7 |
|
| |

(PDF Version of PowerPoint of Presentation) |
 |
| Culex Mosquito |
 West Nile Virus West Nile Virus |
The WNV is mosquito-borne, and infects an astonishingly wide variety of wildlife and domestic animals, in addition to occasionally causing disease in humans (Hayes, 2001). Because of its promiscuous biology, WNV is able to spread rapidly from sylvatic environs (e.g., migrating birds) to domestic animals and humans (McLean et al 2001). The virus was originally identified and named by Smithburn and colleagues in 1937 (Smithburn, et al 1940) while they were working in the West Nile district of Uganda. They were seeking cases of African sleeping sickness. The serum of a 37 year-old woman suffering from fever, and thought to have the protozoan, proved to be negative for that pathogen, but positive for a new viral entity. The virus was subsequently shown to be immunologically related to the Yellow Fever virus family of flavi viruses, all of which can cause encephalitis (St. Louis, Eastern and Western Equine, LaCrosse, Japanese, Kunjin). Development of accurate serological tests in the 1950s allowed epidemiologists to survey for WNV in sera collected from around the world and to conduct serological testing during and after outbreaks of the disease.
2 |
Geographic Distribution of WNV |
|
WNV infection in birds and epidemics in human populations have been recorded from Egypt (Melnick et al, 1951; Taylor et al, 1956), South Africa (McIntosh et al,1976), the Middle East (Katz, et al, 1989), France (Durand et al 2002), Italy (Autorino et al 2002), Greece (Papanagiotou et al 1974), Romania (Ceianu et al 2001), Russia (Platonov 2001), Turkey (Radda 1973), and many African countries (Burt et al 2002).
| |
 |
| (Map provided by CDC) |
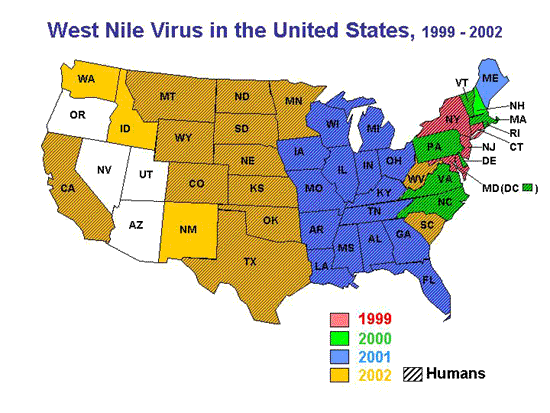
The spread of WNV to the United States in the summer of 1999 was the first description of this virus in the New World (Lanciotti et al, 1999). In that year, during the summer and early fall, it caused illness in 67 individuals, killing 7. One was from Toronto , Canada and that unfortunate individual presumably caught the virus in New York during a brief stop-over while on his way home. Based on prior outbreaks in Africa and Europe , and follow-up serological surveys, it is estimated that for every clinical case, there are at least 150-200 infections that go undiagnosed. Thus, in New York and environs ( Connecticut and New Jersey ), as many as 13,400 people may have become infected in 1999. In addition, numerous species of western hemisphere bird (e.g., song birds, crow, blue jay) became ill and many died. Exotic bird species at the Bronx Zoo (bald eagle, flamingo, bronze duck, etc.) were among its first victims (Ludwig et al 2002). Native birds throughout the greater metropolitan area were dying, as well. Some horses on Long Island became ill, and a few died. Mosquito species that harbored the virus included Culex pipiens, Cx. restuans, Aedes triseriatus, Ae. vexans and Ae. japonicus .( Morb Mortal Wkly Rep. 2002 ).
In 2000, the WNV spread from Connecticut, New York, New Jersey, and Maryland to Vermont, New Hampshire, Rhode Island, Massachusetts, Pennsylvania, Delaware, Virginia, and North Carolina. Despite this rapid movement down the eastern seaboard, WNV was relatively quiescent in the human population, with only 18 cases and some 3,600 infections. In contrast, the virus continued to steadily spread into wildlife, and among bird species in particular. Corbidae (crows and blue jays) emerged as the most susceptible avian family, both in terms of acquisition of infection and sensitivity to its lethal effects (Eidson 2001). Dead crows have since then become the standard indicator for identifying areas of transmission.
In 2001, the virus continued its slow spread westward, killing thousands more birds and infecting an increasingly widening network of wildlife. Human cases numbered 66, with 9 deaths and approximately 13,200 infections (O'Leary et al 2002).
The year 2002 proved to be different from all preceding years. A major epidemic occurred, with 3,852 cases, 263 deaths, and an estimated 700,000 infections ( Morb Mortal Wkly Rep. 2002 ). In addition, 14,358 domestic horses became ill, and 4,300 died. Record numbers of dead birds were reported to state health authorities, mostly crows and blue jays. The causes of death went largely undiagnosed, but most were presumed to be due to the virus. Insecticide poisoning was also suspected in a number of bird deaths, an indirect result of public reaction (i.e., increased individual home use and mis-use of various insecticides) to the threat of acquiring WNV. By the fall, a remarkable number of states (39) and the District of Columbia recorded the presence of the virus in humans, a plethora of wildlife, and domestic animals. States that reported more than 150 cases were Michigan (524), Ohio (434), Louisiana (325), Indiana (294), Mississippi (190), and Texas (190), and Missouri (169). A main feature of that epidemic was heat and drought, now a common theme to almost every recorded outbreak, regardless of location (see: West Nile Story, Despommier, 2000). Many southern and Midwestern states recorded record-breaking temperatures and suffered through one of their worst droughts, that lasted for most of the summer.
 |
| (click on the map to see data, provided by CDC) |
| |
(click on the map to see data, provided by CDC) |
| |
 |
| |
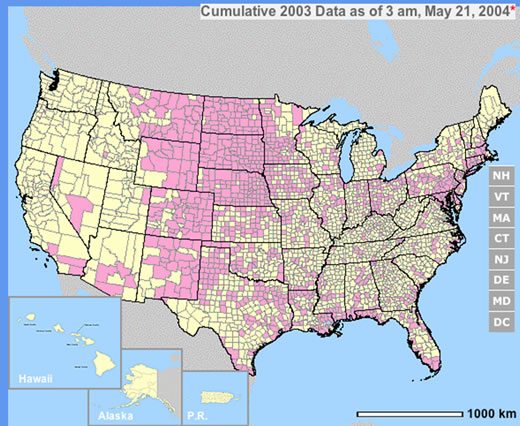
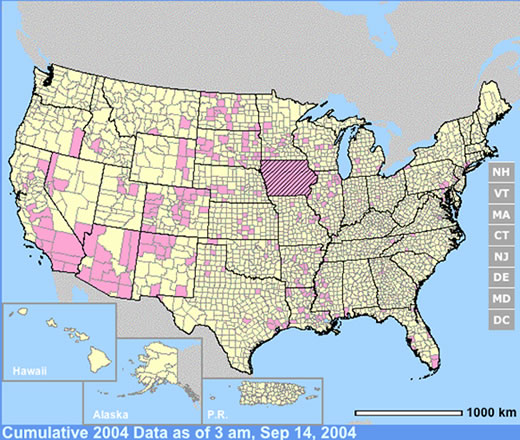
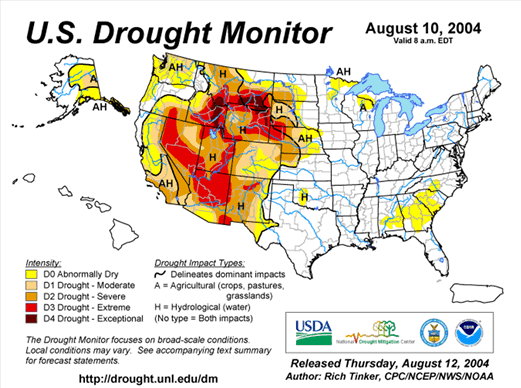
The year 2003 saw WNV still on the march. The number of cases far exceeded the previous year's world record number of hospitalized individuals (9,186 – CDC End of Year Morbidity and Mortality Reports 2003). This represents an astounding potential 1,837,200 infections, a new world record. Most occurred in the states of Colorado (2,477), Nebraska (1,867), and South Dakota (1,039). Again, heat and drought were key to understanding the medical ecology of this epidemic, with that region of the west suffering its worst drought in 100 years. Daily temperatures frequently exceeded 90°F for several days in a row, amplifying the virus in those mosquito species ( Culex tarsalis was the most common one implicated) that were infected.
4 |
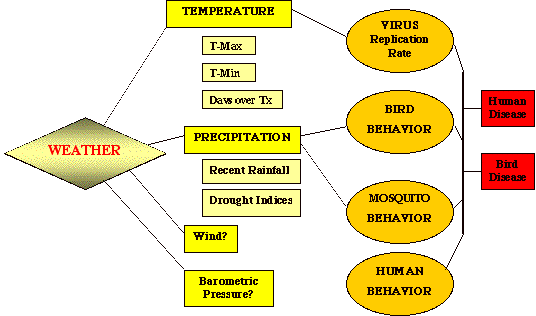
Environmental Aspects of WNV Transmission |
 |
| |
What role(s) does weather, climate, and the landscape play regarding the medical ecology of WNV? An extensive literature documenting outbreaks in Africa, Europe, the Middle East, and Russia (see: West Nile Virus: Detection, Surveillance, and Control. Ann. NY Acad. Sci. Vol. 951. pp. 374) indicates that emergence of WNV in human populations is associated with prolonged periods of hot, dry weather that is often followed by a significant rain event (Epstein 2001). Under these conditions, infection seems to shift from birds to humans. Vector competence for at least some arboviruses ( St. Louis and WNV) is directly related to ambient temperatures (Hurlbut 1973; Dohm et al 2002) and undoubtedly this single has a significant effect on the transmission of WNV. The hotter the temperature, the higher the viral load per infected mosquito, and thus the more likely is the probability that the vector will transmit the agent when taking its next bloodmeal. The main vectors for WNV in birds and people are members of the genus Culex (e.g., Cx. pipiens . Cx. tarsalis , Cx. molestus , and Cx. univittatus ) (Turell et al 2002). Many other species have also been shown to harbor the virus, but their role in transmission of WNV to humans has yet to be determined. Most Culex species are peri-domestic, and prefer to breed in temporary bodies of standing freshwater that are nutrient-enriched (i.e., polluted). This is particularly true for Cx. pipiens , and is considered a pest species of water treatment plants. Cx. pipiens prefers to feed on birds, but will occasionally feed on humans when birds are not available. Such a situation may arise during extended periods of drought. In South Africa in 1974, over 3,000 cases of WNV occurred following a deluge that broke a two-month long drought, the worst dry spell in that region's recorded history (McIntosh et al 1976). Only one species of mosquito, C. univittatus , was implicated in the outbreak, despite intensive surveys to find other candidate vector species. Like C. pipiens , C. univittatus prefers to feed on birds, but will switch to human sources of blood when absolutely necessary.
WNV is endemic in people living in the semi arid regions of southern Egypt and central and northern South Africa . Both regions are desert ecosystems, and the weather most of the year is decidedly hot and dry. In southern Egypt , the infection appears to be restricted to children, with few if any cases occurring in adults (Taylor et al 1956). Presumably, this is because the infection is ubiquitous among the younger age groups, and infection results in permanent immunity. The situation is different in northern Egypt , where the climate and weather patterns are more akin to those typical of southern Europe (ibid). Warm, wet summers are typical, but droughts frequently occur, and when they do, its mostly in late summer and early fall. Outbreaks of WNV routinely occur at that time.
In 1999, New York City recorded the worst drought in 100 years, with temperatures exceeding 100 F at least six times during the month of July (Despommier, 2000). On August 26, 1.58 inches of rain broke the drought, and the rate of infection in humans rapidly accelerated. The epidemic ended in September with the arrival of hurricane Floyd. The following year, 2000, was the wettest and coolest spring and early summer on record over the past 100 years. Few cases of WNV occurred, and the vast majority of those resided in Staten Island .
2002 was also a drought year for most of the eastern half of the United States, with prolonged spells of hot, dry weather recorded in the upper Midwest and South. WNV affected these two geographic regions the most that summer, while the eastern seaboard had only a few human cases. In contrast, birds and other animals continued to accumulate high rates of morbidity and mortality from WNV throughout the entire eastern half of the country. Thus, we speculate that WNV emerges into human populations when a specific pattern of weather occurs; namely hot and dry for more than two weeks.
5 |
Hypothesis:
Ecology of Transmission of WNV to Humans |
|
The following speculation is based on a wealth of observational data collected from various sites of outbreaks over the last 20 years. WNV is primarily an infection of wildlife. When conditions favor transmission from bird to bird (cool, wet summer months), people living in the same transmission zone rarely become infected. That is because the species of mosquitoes most frequently implicated in transmission ( Culex pipiens and its close relatives , Cx. tarasalis, and Cx. molestus ) choose birds to humans as a source of blood for egg laying. When conditions change (i.e., drought and high daily temperatures), several things conspire to reverse the feeding habits of these mosquitoes.
First, birds fly to new, wetter environs when droughts occur, stranding those species of vectors that prefer them to humans. Second, it is known that high temperatures drive up the viral titers in infected mosquitoes. Its possible that by pushing viral titers over a certain threshold, the number of viral particles now exceeds that needed for minimal transmission to people. Apparently, several days worth of 90+°F days are required for this step in the epidemic. Third, most other species of mosquito decrease in numbers due to lack of proper breeding sites. Unfortunately, several Culex species prefer laying their eggs in stagnant, polluted water. Humans are excellent providers of sources of polluted water - unattended swimming pools, bird baths, dripping garden hoses, etc.. Thus we encourage the breeding of these peri-domestic insect vectors by our sloppy behavior. The result is that Culex, faced with a choice of not feeding at all (no birds in the area) or feeding solely on humans, reluctantly feeds on us. This heralds the initiation of an outbreak in the human population. It is also possible that humans become bitten by infected mosquitoes prior to an outbreak, but receive less than the needed minimal infecting dose of virus due to low viral titers associated with low ambient temperatures experienced by the vectors. When the drought eventually breaks, the Cx. spp . spreads out even further, carrying with them the virus to new places in the local vicinity. Eventually, the landscape greens up again, enticing the birds to return. When this occurs, the epidemic in humans ends and the one in birds resumes.
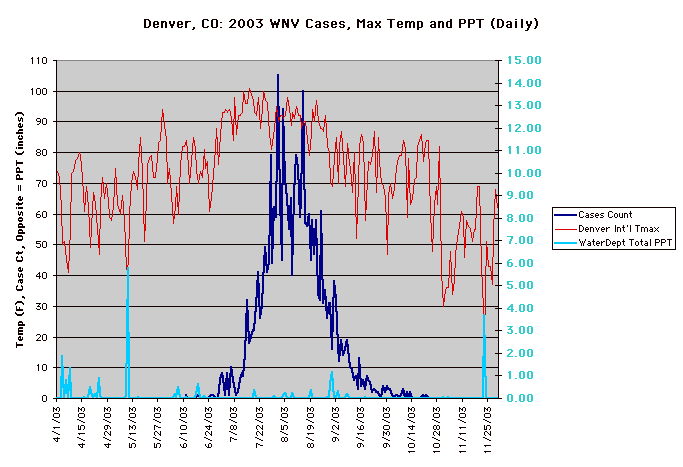
Data collected by Nicole Arshenko
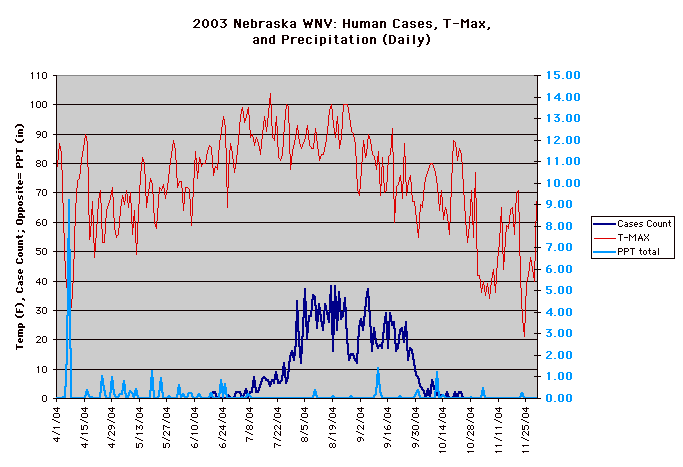
Data collected by Nicole Arshenko
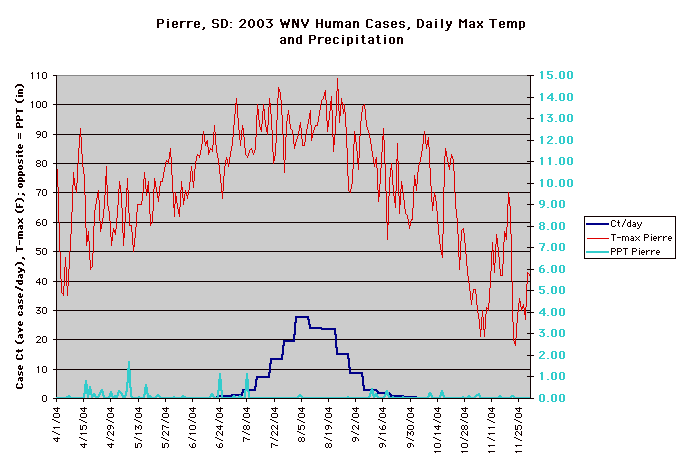
Data collected by Nicole Arshenko
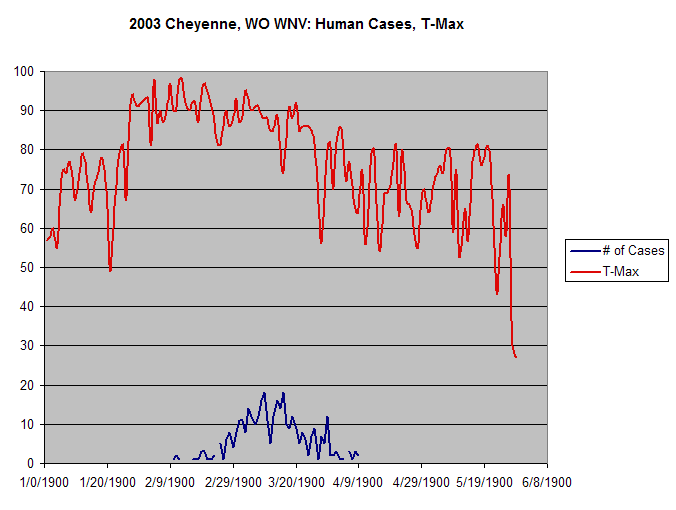
Data collected by Nicole Arshenko
Autorino, G.L., Battista, A, et al. 2002. West Nile virus epidemic in horses, Tuscany region, Italy. Emerg Infect Dis. 8:1372-1378.
Burt, F.J. et al. 2002. Phylogenetic relationships of southern African West Nile virus isolates. Emerg Infect Dis. 8 :820-6.
Ceianu, C.S., Ungureanu A. et al. 2001. West Nile virus surveillance in Romania : 1997-2000. Viral Immunol.14:251-62.
Dohm DJ, O'Guinn ML, Turell MJ. 2002. Effect of environmental temperature on the ability of Culex pipiens (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2002. 39:221-5.
Despommier, D.D. 2000. West Nile Story. Apple Trees Productions, LLC. pp. 145.
Durand B, Chevalier, V. et al. 2002. West Nile virus outbreak in horses, southern France , 2000: results of a serosurvey. Emerg Infect Dis. 8:777-782
Eidson, M. 2001. "Neon needles" in a haystack: the advantages of passive surveillance for West Nile virus. Ann N Y Acad Sci. 951:38-53.
Epstein, P.R. 2001. West Nile virus and the climate. J Urban Health. 2001. 78:367-71.
Hayes, C. G. West Nile Virus: Uganda , 1937, to New York City , 1999. 2001. Ann. NY Acad. Sci. Vol. 951. p. 25-37
Katz, G., Rannon, L et al. 1989. West Nile Fever – Occurrence in a new endemic site in the Negev . Israeli J. Med. Sci. 25: 439-441.
Ludwig G.V., Calle, P.P. et al. 2002. An outbreak of West Nile virus in a New York City captive wildlife population. Am J Trop Med Hyg. 67:67-75.
McIntosh, B.M. Jupp, P.G., et al. 19746.. Epidemics of West Nile and Sinbis viruses in South Africa with Culex (Culex) univittatus Theobald as the vector. S. Afr. J. Sci. 72: 295-300.
McLean, R. G., Sonya, R., et al. 2001. NY Acad. Sci. Vol 951. p. 54-57
Melnick, J.L. Paul, J.R. et al. 1951. Isolation from human sera in Egypt of a virus apparently identical to the West Nile Virus. Proc. Soc. Exp. Biol. Med. 77:661-665.
O'Leary D.R., Nasci, R.S. et al. 2002. From the Centers for Disease Control and Prevention. West Nile Virus activity-- United States , 2001. JAMA. 288:158-159.
Papapanagiotou J. Kyriazopoulou, V. et al 1974. Haemagglutination-inhibiting antibodies to arboviruses in a human population in Greece . Zentralbl Bakteriol 228:443-446.
Platonov, A.E. 2001. West Nile encephalitis in Russia 1999-2001: were we ready? Are we ready? Ann N Y Acad Sci. Vol. 951:102-16.
Provisional surveillance summary of the West Nile virus epidemic-- United States , January-November 2002. Morb Mortal Wkly Rep. 2002. 51:1129-1133
Radda, A. 1973. Studies on the activity and ecology of arboviruses in Turkey . Zentralbl Bakteriol. 225:19-26.
Smithburn, K.C., Hughes, T.P., et al 1940. A neurotropic virus isolated from the blood of a native of Uganda . Am. J. Trop. Med. Hyg. 20: 471-472
Taylor , R.M, Work, T.H., Hurlbut, H. S. et al. 1956. A study of the ecology of West Nile virus in Egypt. Am. J. Trop. Med. Hyg. 5: 5790-620.
Theophilides CN, Ahearn SC , Grady S, Merlino M. 2003 Identifying West Nile virus risk areas: the Dynamic Continuous-Area Space-Time system. Am J Epidemiol. 157:843-54.
Turell, M.J. Sardelis, M.R., O'Guinn, M.L., Dohm, D.J. 2002. Potential vectors of West Nile virus in North America . Curr Top Microbiol Immunol. 2002. 267:241-52.
May 5, 2006 — West Nile In Argentina 
WEST NILE VIRUS, EQUINES - ARGENTINA (03): OIE
Apr 26, 2006
From: ProMED-mail <promed@promedmail.org>
West Nile fever in Argentina
Disease never reported before in Argentina
Translation of information received on 21 Apr 2006 (dated 19 Apr 2006) from
Dr Jorge Nestor Amaya, president, National Agrifood Health and Quality
Service (SENASA), Secretariat for Agriculture, Livestock, Fisheries and
Food, Buenos Aires:
Identification of agent: West Nile fever virus.
Date of start of the event: 4 Feb 2006.
Two outbreaks of West Nile fever have been reported in San Antonio de
Areco, Buenos Aires province. They have occurred in 2 breeding farms of
purebred racehorses with 294 and 331 animals respectively. The dead animals
were an 8 year old and an 18 year old, both mares.
The diagnosis was established on 19 Apr 2006 at the National Institute of
Human Viral Diseases, by Dr Julio I Maistegui.
The control measures undertaken are: quarantine and screening.
Those to be undertaken: control of arthropods. The use of vaccination is
also being considered as a control measure.
In just under 7 years, West Nile virus has swept through the western
hemisphere. It entered New York City in late August 1999 and has now
arrived in Argentina. There is some evidence that it has been in Argentina
since 2004; see references below.
As the virus has become endemic in the United States morbidity in horses
declined dramatically from over 10 000 cases in 2002 to less than 1000
cases in 2005. See graph from USDA-APHIS at <http://www.aphis.usda.gov/vs/ceah/ncahs/nsu/surveillance/wnv/wnv_trends.htm>.
In addition, vaccines have been developed and approved for use in horses,
substantially reducing the risk to horses. While the disease can cause
severe neurological signs and mortality in horses, it can be managed by
mosquito protection and vaccination. Over the years, this disease has, like
any "good disease", behaved well by becoming less of a problem as it
develops endemicity. What, of course, you don't want is a disease that
stays as virulent as when 1st recognized, one that becomes worse over time
or for which we can't develop any tools of control.
More details of the outbreak will appear in the Weekly OIE Disease
Information report, usually issued on Thursday. - Mod.PC]
[see also:
West Nile virus, equines - Argentina (02) 20060426.1216
West Nile virus, equines - Argentina (02) 20060425.1204
West Nile virus, equines - Argentina: RFI 20060423.1196
2005
---
West Nile virus update 2005 - Western Hemisphere (22) 20051226.3686
West Nile virus, humans, equines - Cuba 20050202.0355
West Nile virus update 2005 - Western Hemisphere (01) 20050106.0033
2004
---
West Nile virus update 2004 - Western Hemisphere (01) 20040730.2073
West Nile virus update 2004 - Western Hemisphere (29) 20041231.3456
West Nile virus, birds - Puerto Rico (Ceiba) 20040614.1599
2003
---
West Nile virus - Caribbean Islands (02) 20031017.2619
West Nile virus - Caribbean Islands 20031011.2560
West Nile virus, equines - El Salvador 20030504.1116
West Nile Virus, birds, migratory - Americas 20031007.2512
West Nile Virus, birds, migratory - Americas (02) 20031009.2545
West Nile virus, birds - Dominican Republic 20030315.0645
2002
---
West Nile virus, human, - (El Salvador): suspected 20020909.5264
2001
---
West Nile virus, human - Cayman Islands 20011016.2538]
April 4, 2006
West Nile Virus Epidemics in North America Are Driven by Shifts in Mosquito Feeding
Behavior
— PLoS Biology, April 2006, Volume 4, Issue 4

(PDF Presentation)
WEST NILE VIRUS UPDATE 2005 - WESTERN HEMISPHERE (22)
Date: Tue 20 Dec 2005
Source: USA Centers for Disease Control and Prevention, Division of
Vector-borne Infectious Diseases, West Nile virus, 20 Dec 2005
<http://www.cdc.gov/ncidod/dvbid/westnile/surv&control05Maps.htm>
<http://www.cdc.gov/ncidod/dvbid/westnile/surv&controlCaseCount05_detailed.htm>
US CDC/ArboNET Report (2005): 2005 West Nile virus activity in the
United States (reported to CDC as of 20 Dec 2005)*
*Currently, West Nile virus (WNV) maps are updated weekly to reflect
surveillance reports released by state and local health departments
to CDC's ArboNET system for public distribution.
As of 20 Dec 2005, avian, animal or mosquito WNV infections have been
reported to CDC ArboNET from the following states: Alabama, Arizona,
Arkansas, California, Colorado, Connecticut, Delaware, District of
Columbia, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kansas,
Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan,
Minnesota, Mississippi, Missouri, Montana, Nebraska, Nevada, New
Hampshire, New Jersey, New Mexico, New York, North Carolina, North
Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South
Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Virginia,
Washington, West Virginia, Wisconsin, and Wyoming. [The status has
not changed for the past 2 postings, i.e. a total of 48 states and
one special district, that is DC.]
Human cases have been reported in: Alabama, Arizona, Arkansas,
California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho,
Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maryland,
Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana,
Nebraska, Nevada, New Jersey, New Mexico, New York, North Carolina,
North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island,
South Carolina, South Dakota, Tennessee, Texas, Utah, Wisconsin, and
Wyoming [a total of 42 states].
Maps detailing county-level human, mosquito, veterinary, avian and
sentinel data are published each week on the collaborative USGS/CDC
West Nile virus website: <http://westnilemaps.usgs.gov/>
State / Neuroinvasion* / West Nile fever** / Other*** / Total**** / Deaths
-----------------------------------------------
Alabama / 6 / 4 / 0 / 10 / 2
Arizona / 44 / 47 / 19 / 110 / 4
Arkansas / 11 / 15 / 0 / 26 / 3
California / 283 / 509 / 73 / 865 / 18
Colorado / 20 / 81 / 0 / 101 / 2
Connecticut / 4 / 2 / 0 / 6 / 1
Delaware / 1 / 0 / 1 / 2 / 0
Florida / 9 / 12 / 0 / 21 / 1
Georgia / 9 / 7 / 4 / 20 / 1
Idaho / 2 / 7 / 4 / 13 / 0
Illinois / 132 / 88 / 27 / 247 / 13
Indiana / 10 / 2 / 11 / 23 / 1
Iowa / 13 / 21/ 3 / 37 / 2
Kansas / 13 / 6 / 0 / 19 / 1
Kentucky / 5 / 0 / 0 / 5 / 1
Louisiana / 100 / 38 / 0 / 138 / 8
Maryland / 4 / 1 / 0 / 5 / 0
Massachusetts / 4 / 2 / 0 / 6 / 1
Michigan / 36 / 5 / 14 / 55 / 4
Minnesota / 17 / 27 / 0 / 44 / 3
Mississippi / 39 / 31 / 0 / 70 / 6
Missouri / 17 / 14 / 0 / 31 / 2
Montana / 8 / 17 / 0 / 25 / 0
Nebraska / 43 / 90 / 0 / 133 / 2
Nevada / 14 / 15 / 2 / 31 / 0
New Jersey / 3 / 3 / 0 / 6 / 0
New Mexico / 20 / 13 / 0 / 33 / 2
New York / 10 / 4 / 0 / 14 / 2
North Carolina / 2 / 2 / 0 / 4 / 0
North Dakota / 12 / 74 / 0 / 86 / 0
Ohio / 46 / 15 / 0 / 61 / 2
Oklahoma / 16 / 14 / 0 / 30 / 1
Oregon / 1 / 6 / 0 / 7 / 0
Pennsylvania / 14 / 11 / 0 / 25 / 0
Rhode Island / 1 / 0 / 0 / 1 / 0
South Carolina / 5 / 0 / 0 / 5 / 1
South Dakota / 35 / 192 / 1 / 228 / 2
Tennessee / 14 / 3 / 0 / 17 / 1
Texas / 107 / 51 / 0 / 158 / 10
Utah / 21 / 31 / 0 / 52 / 1
Wisconsin / 11 / 6 / 0 / 17 / 2
Wyoming / 6 / 6 / 0 / 12 / 2
Totals: 1168 / 1472 / 159 / 2799 / 102
---------------------
* Cases with neurologic manifestations (such as WN meningitis, WN
encephalitis, WN myelitis)
** Cases with no evidence of neuroinvasion
*** Illnesses for which insufficient clinical information was provided
**** Total number of human cases of WNV illness reported to ArboNET
by state and local health departments.
Total Human Cases Reported to CDC: These numbers reflect both mild
and severe human disease cases occurring between 1 Jan 2005 through
20 Dec 2005 that have been reported to ArboNet by state and local
health departments. ArboNet is the national, electronic surveillance
system established by CDC to assist states in tracking West Nile
virus and other mosquito-borne viruses. Information regarding 2005
virus/disease activity is posted when such cases are reported to CDC.
Of the 2799 cases, 1168 (42 percent) were reported as West Nile
meningitis or encephalitis (neuroinvasive disease), 1472 (53 percent)
were reported as West Nile fever (milder disease), and 159 (6
percent) were clinically unspecified at this time. Please refer to
state health department web sites for further details regarding state
case totals. Note: The high proportion of neuroinvasive disease cases
among reported cases of West Nile virus disease reflects surveillance
reporting bias. Serious cases are more likely to be reported than
mild cases. Also, the surveillance system is not designed to detect
asymptomatic infections. Data from population-based surveys indicate
that, among all people who become infected with West Nile virus
(including people with asymptomatic infections), less than one
percent will develop severe neuroinvasive disease. See: Mostashari F,
Bunning ML, Kitsutani PT, et al. Epidemic West Nile encephalitis, New
York, 1999: results of a household-based seroepidemiological survey.
Lancet 2001; 358: 261-4.
see also:
West Nile virus update 2005 - Western Hemisphere (21) 20051212.3573
West Nile virus update 2005 - Western Hemisphere (20) 20051202.3477
West Nile virus update 2005 - Western Hemisphere (19) 20051113.3320
West Nile virus update 2005 - Western Hemisphere (18) 20051106.3248
West Nile virus update 2005 - Western Hemisphere (17) 20051030.3161
West Nile virus update 2005 - Western Hemisphere (16) 20051019.3044
West Nile virus update 2005 - Western Hemisphere (15) 20051009.2946
West Nile virus update 2005 - Western Hemisphere (14) 20051001.2870
West Nile virus update 2005 - Western Hemisphere (13) 20050917.2748
West Nile virus update 2005 - Western Hemisphere (12) 20050910.2685
West Nile virus update 2005 - Western Hemisphere (11) 20050903.2608
West Nile virus update 2005 - Western Hemisphere (10) 20050826.2522
West Nile virus update 2005 - Western Hemisphere (09) 20050823.2481
West Nile virus update 2005 - Western Hemisphere (08) 20050809.2323
West Nile virus update 2005 - Western Hemisphere (07) 20050805.2279
West Nile virus update 2005 - Western Hemisphere (06) 20050718.2070
West Nile virus update 2005 - Western Hemisphere (05) 20050702.1869
West Nile virus update 2005 - Western Hemisphere (04) 20050626.1793
West Nile virus update 2005 - Western Hemisphere (03) 20050305.0670
West Nile virus update 2005 - Western Hemisphere (02) 20050215.0503
West Nile virus update 2005 - Western Hemisphere (01) 20050106.0033
West Nile virus update 2004 - Western Hemisphere (29) 20041231.3456
West Nile virus update 2004 - Western Hemisphere (12) 20040820.2312
West Nile virus update 2004 - Western Hemisphere (01) 20040608.1542]
[Back To Top] |
|