| |
Prepared in collaboration with Stephen Schachterle
Outline of Sections
Section 1 |
|
1.1 |
|
1.2 |
|
1.3 |
|
Section 2 |
|
2.1 |
|
2.2 |
|
2.3 |
|
2.4 |
|
2.5 |
|
2.6 |
|
Section 3 |
|
|
|
3.2 |
|
3.3 |
|
3.4 |
|
1.1 |
What is Lyme disease? |
|
|
Borrelia burgdorferi (dark field)
All Borrelia spp. measure 10-30 μm in length. |
| |
| |
Three gram-negative bacteria (spirochetes), Borrelia burgdorferi, B. afzelii, and B. garinii are the most common infectious agents of Lyme disease. Like all spirochetes, Borrelia spp. are slender and corkscrew in morphology. Bites from ticks, primarily of the genus Ixodes, routinely transmit Borrelia spp. from mammal to mammal. In humans, shortly after a primary infection, the organism elicits a red disk-shaped rash, referred to as erythema migrans (EM), at the site of the bite. Infections can be asymptomatic for years. Long-term complications include arthritis-like joint disease and disorders of the nervous system and the heart. Borrelia spp. parasitize different organs depending on the age of the victim and the species of spirochete. Borrelia spp. have a wide range of reservoirs, including lizards, birds, and mammals—particularly rodents. The white-footed deer mouse, Peromyscus leucopus, and the white-tailed deer, Odocoileus virginianus, are the dominant reservoirs in the Eastern United States. In Europe and Asia, Borrelia spp. infect a wider range of animals, none of which are dominant, due largely to the lack of large populations of single mammalian species.
|
|
Scanning electron micrograph of Borrelia burgdorferi
(false color)
|
Bull's eye rash |
1.2 |
What is the geographical distribution of Lyme disease? |
|
Lyme disease has been diagnosed throughout North America, Europe, and Asia (although it may exist undiagnosed elsewhere). Recent evidence suggests that Lyme disease may even occur in Cuba. Dr. Islay Rodríguez and his colleagues described a cohort of Cuban patients with positive serological tests for Lyme disease and all the symptoms of Lyme disease including the characteristic EM. Except for the characteristic “bull’s eye” rash, most other signs and symptoms of Lyme disease are nonspecific and occur over a long period of time, making diagnosis difficult without laboratory confirmation.
1.3 |
When did Lyme disease first appear in the United States? |
|
In all likelihood, Lyme disease has existed in North America and Europe for millennia, however DNA from B. burgdorferi has been identified from mice preserved in museums only as far back as the 19th century. Erythema migrans syndrome, later recognized as Lyme disease, was first seen in Europe during the 1930s. Doctors were able to link the rash to Ixodes tick bites, but the infectious agent remained unidentified. In 1975, an epidemic broke out in Lyme, Connecticut, of what was thought to be childhood arthritis. The outbreak caught the attention of Dr. Allan Steere at Tufts University Medical School in Boston. EM preceded the arthritis in the children, which prompted Steere to look for and find large numbers of Ixodes ticks in the forests and open fields of that region of the Northeastern United States. In 1985, Dr. Andrew Spielman and colleagues at the Harvard University School of Public Health identified the white-footed deer mouse, Peromyscus leucopus, as the dominant mammalian reservoir host for B. burgdorferi.
Dr. Willy Burgdorfer of The National Institutes of Health at the Rocky Mountain Spotted Fever Laboratory in Hamilton, Montana, added to Steere’s original findings in 1982 by extracting the Lyme disease spirochete from Ixodes scapularis. Burgdorfer showed that the pathogens causing the disease in Lyme, Connecticut and the EM syndrome in Europe were one-in-the-same. The infectious agent Borrelia burgdorferi bears his name, honoring him for the discovery.
|
|
|
White footed deer mouse |
Scanning electron micrograph of adult Ixodes scapularis (courtesy David Scharf). Note elongated mouthparts. |
Larva, Nymph, Adult Male, Adult Female (from left to right) |
By 2000, Lyme disease had become the most commonly reported vector borne disease in the United States, with over 18,000 cases recorded by The Centers for Disease Control and Prevention. In Europe, Lyme disease is also the most common vector borne disease. It is endemic in the fragmented forest ecosystems of Sweden, Germany, and in all the Eastern European nations. Cases also occur, in Western Europe, but with much less frequency. In 1995, Slovenia and Austria had incidence rates of 120-130 per 100,000, a rate similar to that found in Lyme, CT.
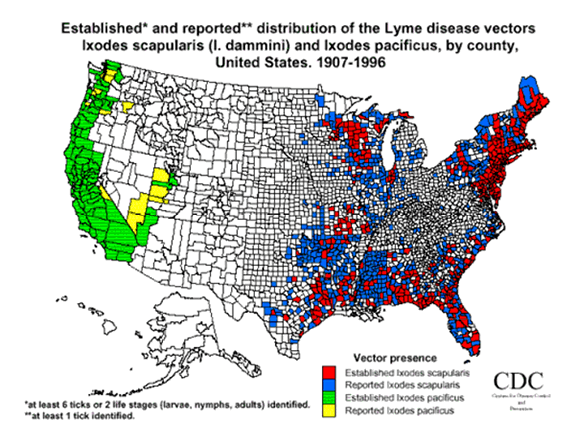
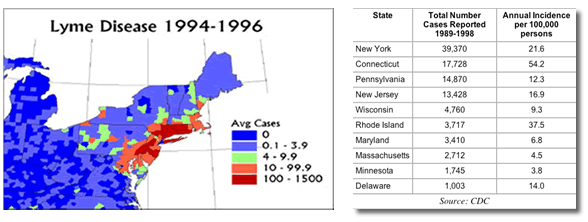
| |
|
2 |
Infectious Agents, Tick Vectors, Reservoirs, and Humans |
|
As stated, bacteria of the genus Borrelia are the cause of Lyme disease. Borrelia spp. are relatives of Treponema pallidum, the infectious agent that causes syphilis. Both kinds of bacteria are spirochetes, and both cause an initial skin irritation followed by long-term systemic (e.g., neurologic) manifestations.
Bacterial Classification Scheme:
| Eubacteria |
Cyanobacteria (blue-green algae) |
|
|
| |
Cytophagales/Green sulfur bacteria group
e.g., Chlorobium |
|
|
| |
Firmicutes (gram-positive bacteria) |
|
|
| |
Actinomycetes - e.g., Clavibacter |
|
|
| |
Proteobacteria (gram negative bacteria is not a taxonomicallyh valid division any more) |
alpha subdivision |
e.g., Rhizobiaceae (rhizobacteria) including Agrobacterium
Bradyrhizobium group
|
| |
|
beta subdivision |
e.g., Burkholderia |
| |
|
gamma subdivision |
e.g., Pseudomonas (fluorescent pseudomonads), Xanthomonas Enterobacteria e.g., Erwinia, Escherichia |
| |
Spirochaetales |
Spirochasetaceae |
Borrelia (Lyme disease) |
In the tick vector, B. burgdorferi lives in the lumen of the mid-gut of the insect. During the act of feeding, B. burgdorferi burrows through the mid-gut mucosa and migrates to the tick’s salivary glands. The tick injects saliva with anticoagulants as it feeds, and B. burgdorferi is injected along with those secretions into the new host. B. burgdorferi expresses different lipoproteins throughout its journey from the tick to its new warm-blooded environment. Temperature plays a major role in mediating these phenotypic changes. Vertical transmission between female ticks and their progeny occurs with regularity, and transmission to over 9 consecutive generations have been documented. This feature of the infection is crucial to its maintenance in the environment.
B. burgdorferi possesses a single linear chromosome, 9 linear plasmids, and 12 circular plasmids. The genetic information for an immuno-dominant outer protein is found on one of the plasmids. Neither chromosomes nor plasmids encode for toxins. B. burgdorferi’s pathogenicity is largely due to its penchant for tunneling through tissue, adhering to cells, and stimulating autoimmune responses. The bacteria can form a cyst to aid in evading the host’s protective immune responses, and can survive in nutrient-poor tissues, such as cerebrospinal fluid, for extended periods of time.
The citation for the complete genome of B. burgdorferi is given below:
Nature 390, 580 – 586.1997.
Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi
CLAIRE M. FRASER*, SHERWOOD CASJENS†, WAI MUN HUANG†, GRANGER G. SUTTON*, REBECCA CLAYTON*, RAJU LATHIGRA‡, OWEN WHITE*, KAREN A. KETCHUM*, ROBERT DODSON*, ERIN K. HICKEY*, MICHELLE GWINN*, BRIAN DOUGHERTY*, JEAN-FRANCOIS TOMB*, ROBERT D. FLEISCHMANN*, DELWOOD RICHARDSON*, JEREMY PETERSON*, ANTHONY R. KERLAVAGE*, JOHN QUACKENBUSH*, STEVEN SALZBERG*, MARK HANSON‡, RENE VAN VUGT†, NANETTE PALMER†, MARK D. ADAMS*, JEANNINE GOCAYNE*, JANICE WEIDMAN*, TERESA UTTERBACK*, LARRY WATTHEY*, LISA MCDONALD*, PATRICIA ARTIACH*, CHERYL BOWMAN*, STACEY GARLAND*, CLAIRE FUJII*, MATTHEW D. COTTON*, KURT HORST*, KEVIN ROBERTS*, BONNIE HATCH*, HAMILTON O. SMITH* & J. CRAIG VENTER*
The blacklegged tick, Ixodes scapularis, is the most common Lyme disease vector in Northeastern and Central North America, while I. pacificus is responsible for B. burgdorferi transmission along the West Coast of The United States. In Europe, the sheep tick, I. ricinis, transmits all three Borrelia spp; while in Asia, the taiga tick, I. persulcatus transmits both B. garinii and B. afzelii.
Ixodes scapularis also can transmit Babesia microti and Anaplasma phagocytophilum. These protozoan infections are usually asymptomatic unless the patient is co-infected with Borrelia, or has had a splenectomy. Co-infection with any combination of either of these two organisms can exacerbate “flu-like” symptoms that accompany Lyme disease. In addition the bactrium Ehrlichia chaffeensis, can cause serious disease when co-infected with B. burgdorferi. Multiple infections with any three of these pathogens is not infrequent in areas of high endemicity.
 |
|
“Questing” Ixodes tick
(Photograph by: Michael Patnaude, University of Florida) |
|
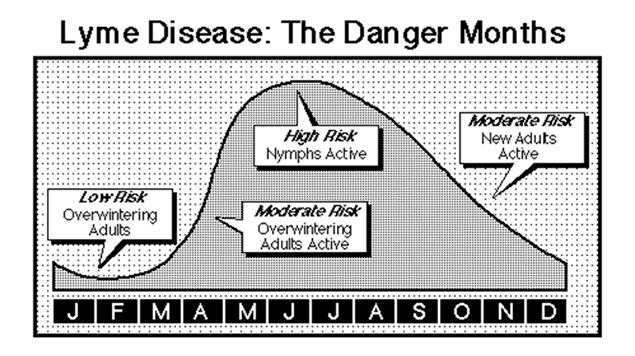
Understanding the biology of I. scapularis is crucial to assessing Lyme disease risk in The United States, since up to 100% of the ticks in any forested ecosystem in the Northeastern portion of that country have been shown to carry the Lyme disease agent. I. scapularis has a year and a half long life cycle. The larval ticks hatch in the summer and feed on their first host, the white-footed deer mouse. Larval I. scapularis are thought to acquire B. burgdorferi vertically from an infected female, and remain infected for life. I. scapularis larvae molt into nymphs and remain dormant throughout the fall and winter. The nymphs re-emerge in the late spring and spend the remainder of the spring and summer searching of another blood meal. The ticks climb up stalks of tall grass and low lying bushes and begin “questing.”
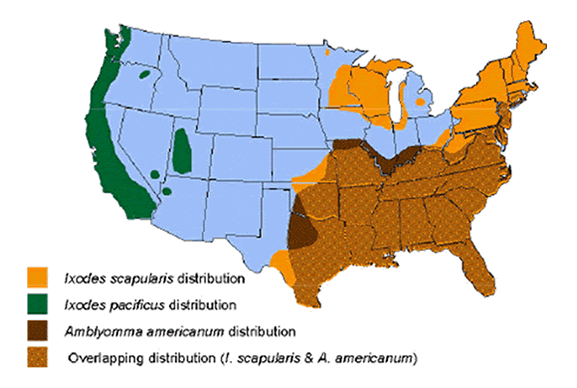
CDC Map
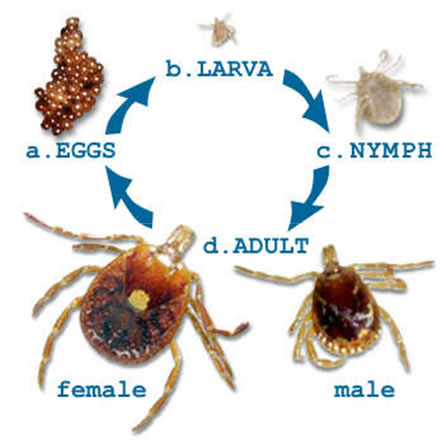
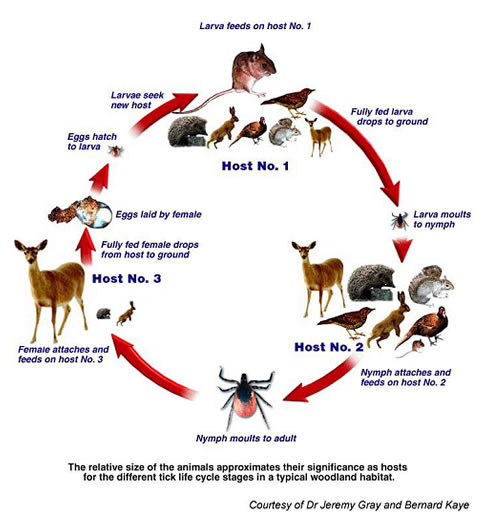
I. scapularis nymphs are most likely to transmit the infection to humans, since they quest in taller vegetation than the larave. If they find a second blood meal, regardless of the species of host, the nymph will molt into the adult stage and begin their “quest” for the final host, the white-tailed deer. Adult ticks that find their way onto deer take their third blood meal, and by doing so achieve reproductive competency. Mating then ensues. Fertilized females drop off the deer, become dormant for the winter and lay their eggs in the early spring of the following year. If the female tick happens to be infected, then all of her offspring will be infected, as well.
The white-footed deer mouse, Peromyscus leucopus, is the most competent rodent reservoir in Eastern North America. The most common large mammal reservoir host is the white-tailed deer.
|
|
White-footed Deer Mouse |
White-tailed Deer |
| |
|
2.4 |
Ecological Studies on Lyme Disease |
|
The two diagrams below illustrate the complexity of attempting to understand and integrate all relevant environmental factors that play a role in the yearly transmission cycle of Lyme disease, both for wildlife and for humans. What follows is a summary of recent ecological studies conducted in the United States that address some of these global issues; in particular, changes in the patterns of weather and competing reservoir host species.
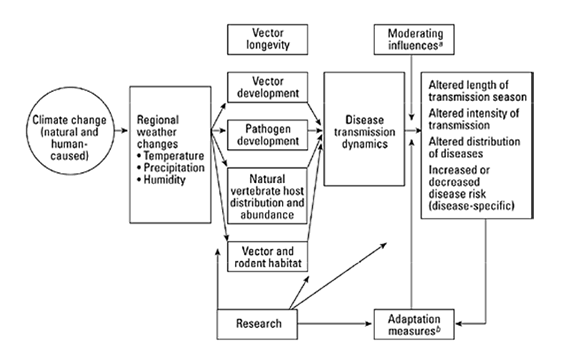
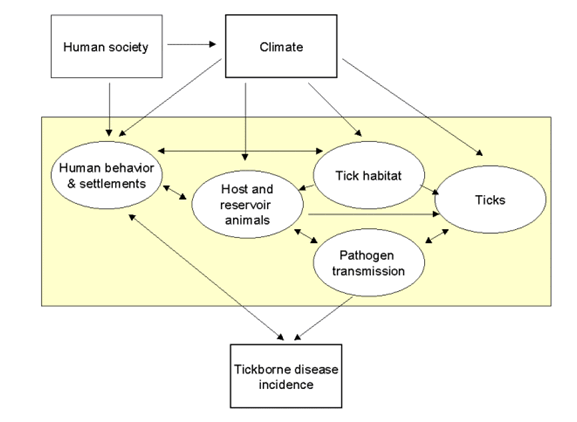
Environmental conditions (e.g., annual precipitation, temperature, fragmentation of the landscape) vary from day to day and from year to year, sometimes significantly affecting the biology of deer, deer mice, and other reservoir host species. As the result, the population of tick vector species also varies, ultimately altering the incidence and prevalence of Lyme disease in human populations in any given area. A few selected studies conducted at the Institute of Ecosystem Studies in Millbrook, New York by Dr. Richard Ostfeld and colleagues will serve to illustrate these points.
The amount of annual precipitation influences the abundance of acorn production in Northeastern hardwood forests, where oaks comprise a dominant portion of the tree population. The abundance of acorns influence white-footed deer mouse populations, impacting on disease prevalence in ticks and disease risk for humans.
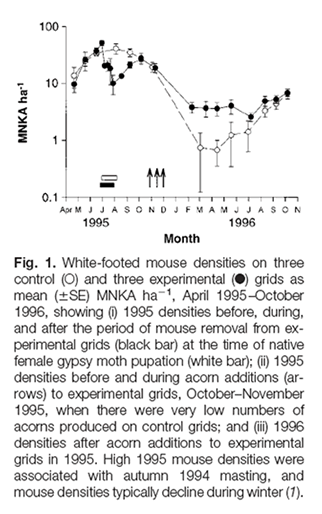
Oak tree patches produce few acorns some years and bumper crops of acorns in others, depending on the amount of annual rainfall. Years of high acorn yields are referred to as mast years. In mast years, white-footed deer mice essentially have an unlimited food supply. They consume acorns during the fall and store them in underground caches as winter food reserve. These mice survive the winter in much higher numbers than normal and are able to breed, since they have adequate food reserves. In non-mast years, without sufficient acorn reserves, mice endure a lean and non-reproductive winter. Research has shown that in the summer following a mast year, mouse populations peak. Ostfeld found that crowding in an experimentally manipulated oak patch forces mice to migrate throughout the forest. Ostfeld hypothesized that nymphal ticks could then infect many more mice with B. burgdorferi. The following year, the large mouse population, now carrying B. burgdorferi, may infect ticks in greater numbers, potentially raising the risk for the disease in human populations living adjacent to those forested areas. Hikers and campers visiting the region would also be at greater risk for acquiring Lyme disease.
The oak masting cycle also influences the behavior of the white-tailed deer. In non-mast years, deer prefer the woody browse found in young forests and at the ecotone between the forests and open fields (e.g., grass lawns, golf courses, etc.). However, when acorns are abundant, deer move into oak patches to feed on them. If the deer are carrying ticks (as is the usual case), they bring them to the oak patch, as well. Ticks then fall off deer, lay their eggs in the leaf litter, and the next generation of ticks (now larvae) encounter large numbers of mice, who were also drawn to the oak patch by the abundance of acorns. Ostfeld and collaborators experimentally added 1 million acorns to a 2.25 hectare swathe of oak-free woods simulating a mast year. They observed a 10 fold increase in larval ticks the following summer as compared to a patch without additional acorns. However, deer exclusion and elimination experiments suggest that reducing deer is an inefficient means of controlling Lyme disease risk in humans. These studies found that near total elimination of deer populations was required to lower risk. Hence, contrary to popular belief, limiting deer populations through recreational hunting will not significantly reduce the risk of acquiring the infection.
The original citation and abstract is listed below:
Ostfeld RS, Schauber EM, et al. 2001. Effects of acorn production and mouse abundance on abundance and Borrelia burgdorferi infection prevalence of nymphal Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 1:55-63.
Risk of exposure to Lyme disease is a function of the local abundance of nymphal Ixodes ticks that are infected with the etiological agent, the spirochete Borrelia burgdorferi. We monitored abundance of white-footed mice (the principal B. burgdorferi reservoir in the eastern and central United States) and acorns (a critical food resource for mice), and Ixodes scapularis ticks, as well as ambient temperature (cumulative growing degree days) and growing season precipitation, in a forested landscape of southeastern New York State from 1994 to 2000. We found that acorn production in autumn strongly influenced abundance of white-footed mice the following summer and that abundance of mice in summer, when larval ticks are active, influenced the abundance of infected nymphs the following year. Consequently, the abundance of infected nymphal ticks can be predicted from acorn production 1.75 years earlier. Monitoring of natural fluctuations in acorn production thus supports results of prior acorn addition experiments that were conducted at small spatial scales. Growing degree days and precipitation either had no significant effect on density of nymphs or marginally increased the explanatory power of models that included acorns or mouse density as independent variables. We conclude that, at our study site in New York, the risk of human exposure to Lyme disease is affected by mouse density in the prior year and by acorn production 2 years previously.
While the white-footed deer mouse and white-tailed deer are the primary targets for Ixodes ticks in eastern North America, along the North American west coast, the wood rat is the most popular Lyme disease reservoir. Ixodes ticks inhabit the American Southwest, as well, but feed primarily on lizards, since there are fewer numbers of mammals in the desert. Fortunately, B. burgdorferi does not survive in lizards and the cycle of transmission is broken. In Europe, one of the principle vectors, I. ricinis feeds on over 300 different vertebrates, including large and small mammals, birds, and reptiles. I. persulcatus, the Asian tick vector, feeds primarily on voles and shrews in the nymphal stage, and hare, deer, cattle, horses, and other larger animals in the adult stage.
Declines in mouse populations tend to reduce disease prevalence in ticks. In a study conducted by Lindsay and colleagues, it was shown that deer mice cannot harbor the infection long enough to allow the infection to over-winter. Apparently, protective immune responses limit the survival of the bacterium in the mice. Infected nymphal ticks can harbor B. burgdorferi for longer periods, and so these immature vectors probably are responsible for initiating most new infections in the spring.
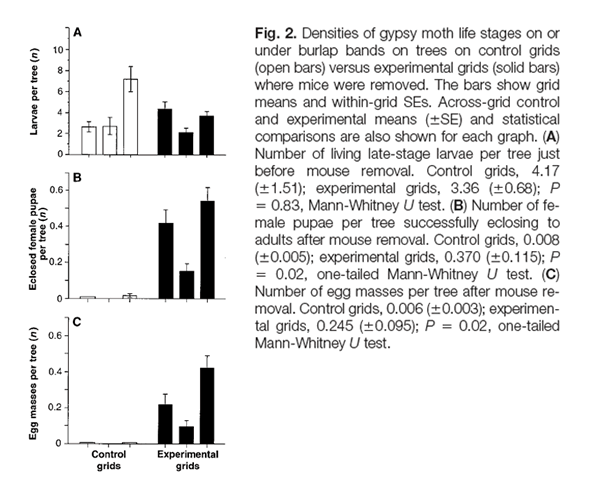
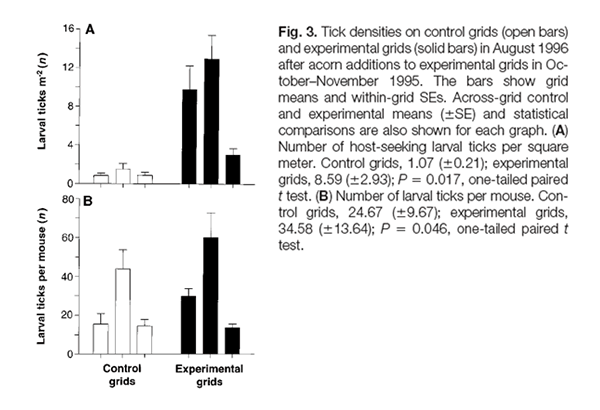
In another study, Ostfeld’s group revealed a correlation between outbreaks of the gypsy moth (Lymantria dispar), and the production of acorns. They found a complex relationship between deer mice, gypsy moth pupae, and acorn abundance. The citation, abstract, and data are shown below:
Jones CG, Ostfeld RS, et al. 1998. Chain reactions linking acorns to gypsy moth outbreaks and Lyme disease risk. Science, 279:1023-1026.
In eastern U.S. oak forests, defoliation by gypsy moths and the risk of Lyme disease are determined by interactions among acorns, white-footed mice, moths, deer, and ticks. Experimental removal of mice, which eat moth pupae, demonstrated that moth outbreaks are caused by reductions in mouse density that occur when there are no acorns. Experimental acorn addition increased mouse density. Acorn addition also increased densities of black-legged ticks, evidently by attracting deer, which are key tick hosts. Mice are primarily responsible for infecting ticks with the Lyme disease agent. The results have important implications for predicting and managing forest health and human health.
2.5 |
Humans: Manipulation of Environments That Favor the Maintenance of Lyme Disease |
|
Humans are not necessary for Borrelia spp. to complete its life cycle, and with over 90% of white-footed deer mice infected in some areas, humans are considered an incidental host. However, people are still crucial players in the ecology of Lyme disease because of our penchant for reshaping the landscape.
In the Northeastern United States, Europeans and their decedents altered the land in ways that, if the disease were present at all during that time, would actually reduce Lyme disease risk throughout the colonial period (1700s). Farmers clear-cut forests to attempt to grow crops, which had the effect of reducing the amount of white-footed deer mouse and white-tailed deer habitat. They also hunted deer to near elimination, which had the effect of reducing further the likelihood of acquiring of Lyme disease.
However, by the middle of the 20th century, the rich soils and high yield productivity of farming in the Midwestern region of the country forced most Northeastern farmers to abandon their fields, and they promptly reverted back to a mixed hardwood forest (see: http://www.hubbardbrook.org/. for an elegant description of the process of re-forestation). In the second half of the 20th century, development returned to the Northeastern forests, now in the form of suburban encroachment. The suburbanites presence kept large predators like wolves, mountain lions, and coyotes away, and because the suburbanites had no particular interest in deer hunting, populations of white-tailed deer and white-footed deer mice soared.
Today, large populations of deer wander fearlessly throughout the suburbs of New York City, Boston, and Philadelphia, serving as the host for untold numbers of Ixodes ticks. The only predators that still function in that capacity in the peri-domestic ecotones of suburbia are an ever-widening variety of Auto mobilus (A. mobilus var. japanensis; A. mobius var. germanicus; A. mobilus var. detroitiana). Suburban development also tends to fragment hardwood forests into small patches, creating ideal habitat for white-footed deer mouse and white-tailed deer populations. Mice are safe not only from large predators, but also mid-sized mammals like the weasels, owls, and hawks that once preyed upon them. People who maintained their bit of the woods in a savanna-like configuration (open spaces with a tree here and there) have created the ideal ecological habitat for Lyme disease transmission.
Suburbanization also set the stage for an ecological phenomenon known as the “dilution effect.” This effect predicts that high species diversity within the community of reservoir hosts serves to reduce the rate of infection in the vector by diluting the effectiveness of the most competent reservoir, the white-footed deer mouse, in maintaining the infection. This theory assumes that the white-footed deer mouse is more competent in transmitting Borrelia spp. to ticks than other known reservoir species, such as birds, reptiles, and gray and red squirrels. It follows from this assumption that the more numerous the less competent reservoir species there are in a given forest patch, the greater the chances are of ticks feeding on them instead of white-footed deer mice. Under these conditions, ticks would be less likely to acquire the infection. Ultimately, this would have consequences for us, in that less Lyme disease would occur. Hence, if this theory holds any truth at all, maintaining a high degree of biodiversity would benefit us, as well as the wild life. The idea that ecosystem services might result from a high degree of biodiversity is not a new one. The original concept was championed by E.O. Wilson and other eminent ecologists. It is, however, reassuring to see some direct evidence in support for it.
White-footed mice are among the last species to leave a community when biodiversity decreases due to severe fragmentation of an Eastern US mixed hardwood forest ecosystem. As species diversity shrinks from human encroachment, white-footed deer mice become more abundant relative to other tick hosts. Therefore, more ticks feed on mice, more ticks contract Borrelia sp., and more people get Lyme disease.
Ostfeld and his colleagues have rigorously scrutinized the dilution effect both mathematically and empirically and have come up with a convincing argument in favor of their theory. The key question remains as to how widely applicable the dilution effect is to other situations in which a vector borne disease is involved. The West Nile virus in The United States might have a similar ecological framework, as well as African and American trypanosomiasis, and some forms of Leishmania. Ostfeld and Keesing do not have the definitive answer on these other pathogens at the moment, but they have set forth guidelines for applying the dilution effect to them. They are: 1) the vector must be a generalist (i.e., not host-specific), 2) the vector must acquire the pathogen directly from the host, 3) infectivity must vary among host species, and 4) the host which transmits the pathogen most readily must be abundant and resilient to local extinction.
A particularly fascinating aspect of the dilution effect is its implication for conservation biology. Environmentalists, as well as much of the general public, seem to want to help nature maintain the richness of biodiversity of animals and plants in our forests. However the benefits to humans of protecting biodiversity remain debatable. The dilution effect suggests high species-diversity favors human health, proving a direct benefit to anyone who enjoys time spent in the outdoors.
2.6 |
Can predators keep you healthy? |
|
A second, related theory also with implications for conservation biology, postulates that predators limit rodent numbers, rodent numbers limit pathogen numbers, and pathogen numbers in rodents then determine disease incidence in people. If predators control the rodents, then fewer rodents means less infection for us. This aspect raises larger questions of human safety, however, especially when other ecological settings and other diseases are the subjects for debate (e.g., mountain lions, tigers, leopards, and bears).
| |
|
3.2 |
Recommended Readings (Original Literature) |
|
- Steere, AC., Coburn, J., Glickstein, L., The Emergence of Lyme Disease. J Clin Invest. 113: 1093-1101.
- Singh, S.K., Girsckick, H.J. Lyme borreliosis: from infection to autoimmunity. Clin Microbiol Infect. 2004. 10: 598-614.
- Giardina, A.R., Schmidt, K.A., Schauber, E.M., Ostfeld, R.S. Modeling the role of songbirds and rodents in the ecology of Lyme disease. Canad J Zoo. 2000. 78: 2184-2197.
- Ostfeld, R.S., Keesing, F. Biodiversity and disease risk: the case of Lyme Disease. Conserv Biol. 14(3): 722-728.
- Stanek, G. Reflections on the clinical and epidemiological studies presented at the IX international confrence on Lyme Borreliosis and other tick-borne diseases and future directions. Vector-Borne and Zoo Dis. 3: 229-243.
- Rodríguez, I,, Fernández, C., Cinco, M., Pedroso, R., Fuentes, O. Do antiborrelial antibodies suggest Lyme disease in Cuba? Emer Infect Dis. 2004 Sep;10(9):1698-1700.
- Ostfeld, RS. The Ecology of Lyme-disease. Amer Scien. 1997. 85(4): 338-347.
- Ostfeld, R.S., Keesing, F. The function of biodiversity in the ecology of vector-borne zoonotic disease. Canad J Zoo. 2000. 78: 2061-2078.
- Schmidt, K.A., Ostefeld, R.S., Biodiversity and the dilution effect in disease ecology. Ecol. 2001. 82(3): 609-619.
- Jones, C.G., Ostfeld, R.S., Richard, M.P., Schauber, E.M., Wolff, J.O. Chain reactions linking acorns to Gypsy moth outbreaks and Lyme disease risk. Science. 1998. 279 1023-1025.
- Lindsay LR, IK Barker, et al. 1997. Duration of Borrelia burgdorferi infectivity in white-footed deer mice for the tick vector, Ixodes scapularis under laboratory and field conditions in Ontario. J Wildl Dis. 33:766-775
- Duffy, D.C., Cambell, S.R., Clark, D., BiMotta, C., Gurney, S. Ixodes scapularis (Acari: Ixodidae) deer tick mesoscale populations in natural areas: effects of deer area and location. J Med Entomol 31: 152-158.
- Allan, B.F., Keesing, F., Ostfeld, R.S. Effect of forest fragmentation on Lyme disease risk. Conserv Biol. 17(1): 267-272.
- LoGiudice, K., Ostfeld, R.S., Schmidt, K.A., Keesing, F., The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Ecol. 2003. 100(2): 567-571.
- Fraser CM, S Casjens, WM Huang, et al. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 390: 580-586.
- Ostfeld, R. S., and R. D. Holt. 2004. Are predators good for your health? Evaluating evidence for top-down regulation of zoonotic disease reservoirs. Front Ecol. Environ. 2:13-20.
- Rosa PA, Tilly K, Stewart PE. 2005. The burgeoning molecular genetics of the Lyme disease spirochaete. Nat Rev Microbiol. 3:129-4
- Foley JE, Foley P, et al. 2004. Ecology of Anaplasma phagocytophilum and Borrelia burgdorferi in the Western United States. J Vector Ecol. 29:41-50.
- Hayes E. 2003. Lyme Disease. Clin Evid. 8:721-33.
- Humair, P, and L. Gern. 2000. The wild hidden face of Lyme borreliosis in Europe.
- Microbes Infect. 2:915-922.
|
|